
Approval Procedures
General requirements
Researchers are permitted to use the SUBI RHTR biological materials for scientific purposes, according to following requirements:
- Transportation costs are paid by the applicant; upon request, estimates will be provided depending on the shipping company, distance, and weight of the package;
- The applicant must provide a detailed shipping address at the time of the application (including building name and room number) as, according to Customs regulations of the Russian Federation, this address cannot be changed after the application is approved;
- Biomaterial and related information about donors can be used only for the research purposes indicated in the application. The received specimens cannot be transferred to anyone not specifically listed in the application;
- Unused or remaining specimens must be returned to SUBI RHTR after the research is completed, at the applicant’s cost;
- Personal identification data and other protected health information may not be disclosed to the applicant;
- Within 3 months after the end of the project investigator who received the specimens must submit a brief report on results of the work;
- All publications resulting from the work must credit the RHTR and SUBI for providing the specimens. The following phrase is recommended for this purpose: “The biospecimens used in this work were provided by the Radiobiological Human Tissue Repository of the Southern Urals Biophysics Institute in Ozyorsk, Russia”.
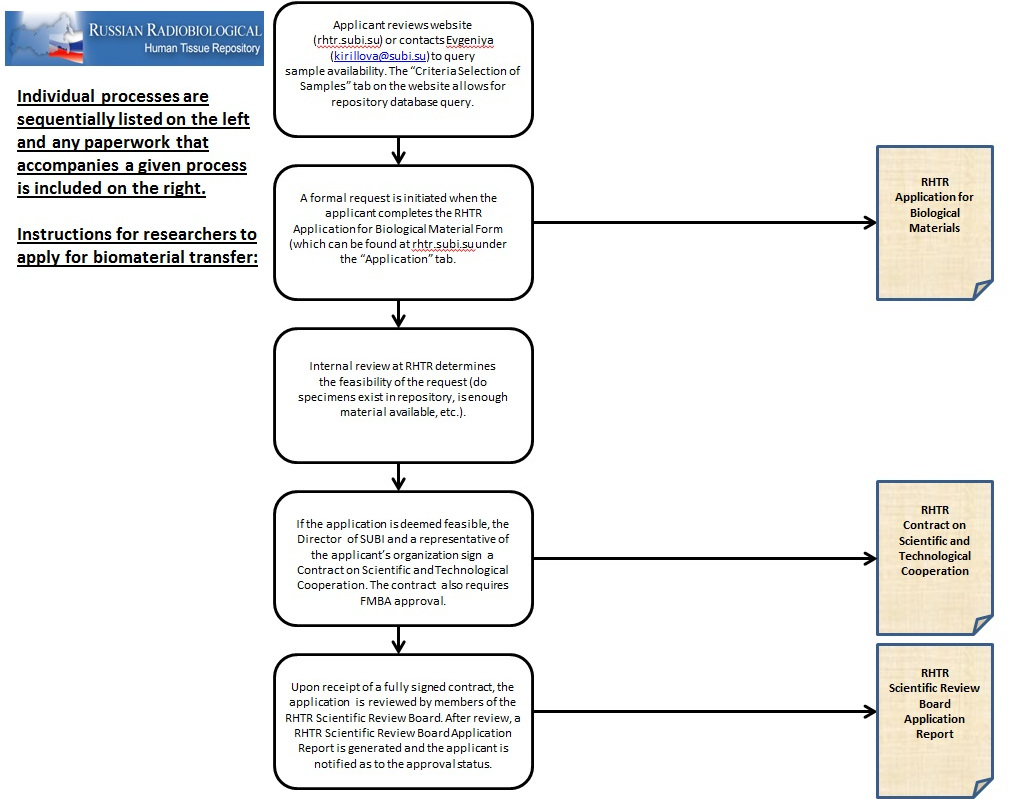
Procedures of application, approval, and transfer of RHTR biological
material
Researchers are permitted to use the SUBI RHTR biological materials for scientific purposes, according to following requirements:
- Biological material can be obtained through formal request and review procedures: the application form can be found under the Application Tab of this web site;
- The completed, signed application should be emailed to the contact persons
listed under the Contact Information Tab on this web site;
Members of the Scientific Review Group involved in the process of approval of requests for biomaterial
USA:
1. Dr. Christopher Loffredo
Professor, Department of Oncology
Professor, Department of Biostatistics, Bioinformatics, and Biomathematics
Lombardi Comprehensive Cancer Center
Georgetown University
Washington, DC, USA2. Jan K. Blancato
Associate Professor , Department of Oncology
Lombardi Comprehensive Cancer Center
Georgetown University USA
Washington, DC, USA.Russia:
1. Aleksandr Biryukov, MD, Professor
The Head of University Department of Clinical and Radiation Epidemiology
The Head of Laboratory for Radiation-Epidemiological Analysis and Clinical Epidemiology
A.I. Burnazyan Federal Medical Biological Centre, Moscow, Russia2. Elena Labutina, PhD, vice-director
Scientific Researcher of the Laboratory of Epidemiology of Late Effects of Radiation Exposure in Workers and Residents
Southern Urals Biophysics Institute (SUBI)
Federal Medical-Biological Agency of Russia (FMBA)
Ozyorsk, Chelyabinsk region, Russia - Decisions about approval of the application for biomaterial transfer will be made within 30 days, according to the following procedures: (a) for applications from members of the SUBI the RHTR manager reviews and recommends approval of the requests; (b) for other Russian researchers, and for researchers outside of the Russian Federation, the application is reviewed and recommended for approval by members of the RHTR Scientific Review Board;
- In the case of a positive decision on biomaterial transfer, the Director of SUBI and a representative of the applicant’s organization (e.g. department chair, Dean or Director, etc…) are required to sign a Contract on Scientific and Technological Cooperation, specifying the conditions for shipment and scientific usage that are listed under General Requirements (above);
- The Contract on Scientific and Technological Cooperation, request for biospecimens and Upon recommendation for approval of the application, it is forwarded to the Director of FMBA of Russia for required signature and final approval.
- Upon receipt of the fully signed Contract, the RHTR arranges shipment of the approved materials to the applicant.